Automobile Alcohols: Methanol and Ethanol

Production
Nikolas August Otto utilized ethanol in his engine prototypes as early as 1860; Henry Ford used bioethanol between 1908 and 1927 in series cars and declared it to be the fuel of the future. Ethanol and methanol are obtained from two types of resources.
Algae and cellulose from waste from the paper and wood-processing industries or from non-edible plants
Sugar cane has been cultivated in Brazil since 1532. Ethanol from sugar cane was being used there as a fuel for car engines between 1925 and 1935. Since 1975, after the first worldwide oil crisis, the Brazilian government has been implementing the national program “Pro Alcohol” with the aim of replacing fossil fuels by alcohols.
The inversion of this tendency back to gasoline had economic and political causes created outside of Brazil. However, this situation was surmounted within a relatively short time. In 2003, the Brazilian VW Golf 1.6 Total Flex was introduced, with an engine that allowed the use of a variable mixture of ethanol and gasoline, in proportions varying between 0 % and 100 %. Seven years later, other cars with flex fuel engines (Chevrolet, Fiat, Ford, Peugeot, Renault, Volkswagen, Honda, Mitsubishi, Toyota, Nissan, and Kia) appeared on the Brazilian market, achieving a proportion of 94 % of new car registrations.
Not only sugar cane, corn, sugar beet, and cassava are utilizable for alcohol production; algae also have a remarkable potential as a resource for alcohol production. Algae are beings that live in water and produce their nourishment by photosynthesis. The earnings per surface, if cultivated in reactors, are much higher than for biomass production in agriculture: 15 times higher than rapeseed and 10 times higher than corn. Research and development activities in the field of production and utilization of algae as fuel are very intensive at this time; Boeing and Exxon are good examples in this sense.
Properties
Methanol and ethanol have lower heat values than gasoline, impairing car autonomy at comparable tank volumes. On the other hand, the lower air/fuel ratio leads to an increase in fuel mass in a mixture for the same air mass within a combustion chamber. Therefore, the heat value of the mixture remains in the range of air–gasoline mixtures.
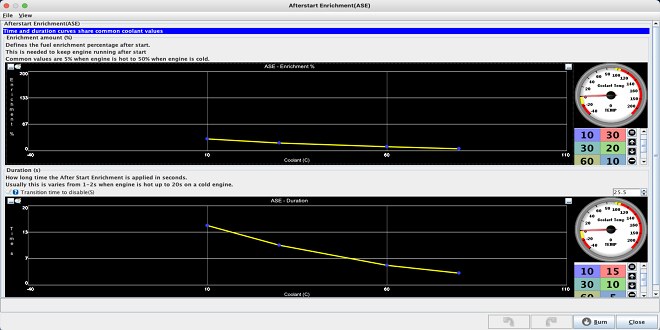
The octane numbers of ethanol and methanol are remarkably higher, allowing an increase in the compression ratio for monovalent engine operation. The vaporization enthalpy is 2.4 times higher than that of gasoline for methanol and times higher for ethanol. This could be an impediment for injection in intake ducts. For direct injection, this high vaporization enthalpy is a net advantage because of the cooling of the compressed air
Storage on Board
Methanol and ethanol have the same density at ambient pressure and temperature as diesel fuel and can be stored in the same kind of reservoir.
Mixture Formation and Combustion
The replacement of gasoline by methanol and ethanol in SI engines with injection in intake ducts was demonstrated in the 1970s, with remarkable success. Porsche tested a fuel containing 85 % methanol and 15 % gasoline, using two fuel pumps instead of one for gasoline, injectors with larger orifices, methanol-resistant materials for all fuel ducts, and methanol-compatible oil.
Last word
Adaptation of the mixture formation and combustion technique to the variable injection rate and injection spray characteristics. At full load, a homogeneous mixture with air is required, whereas at partial load mixture stratification seems to be very beneficial.